New Weekly Injection May Revolutionize Life for Parkinson's Patients

A fresh weekly shot could soon change how countless people handle their condition. Parkinson’s disease Over 8.5 million individuals around the globe who suffer from this disorder must take levodopa and carbidopa pills multiple times each day as part of their regular treatment. Such repeated dosages assist in reducing symptoms such as shaking, rigidity, and slowed movements. However, for older adults and those experiencing trouble swallowing, maintaining this regimen poses challenges and may result in uneven symptom management.
Now, researchers at the University of South Australia (UniSA) has developed a long-acting injection that may make this process simpler by reducing it to a single weekly dose. Their latest formula, described in the journal Pharmaceutical Delivery and Applied Research Medication Administration and Practical Studies Therapeutic Substance Distribution and Innovation Research Clinical Drug Transfer and Developmental Investigation Treatment-Related Compound Transport and Experimental Study Systemic Medication Provision and Translational Exploration Bioavailability Optimization and Clinical Application Research Nanomedicine Deployment and Real-World Implementation Studies Targeted Pharmaceutical Transmission and Scientific Translation Projects Medical Agent Utilization and Evidence-Based Research Approaches integrates scientific innovation, safety features, and user convenience within one long-acting implant.
A weekly injection that delivers medicine gradually
The latest injection includes levodopa and carbidopa—essential medications for managing Parkinson's disease symptoms. After being administered beneath the skin or into the muscle, the biodegradable gel gradually dispenses both medicines over a period of seven days. This steady and controlled release maintains more uniform medication levels in the bloodstream, minimizing adverse effects associated with fluctuations in drug concentration.
Leading researcher Professor Sanjay Garg, affiliated with UniSA's Centre for Pharmaceutical Innovation, believes this development may transform the management of Parkinson's disease. "Our objective was to develop a formula that makes therapy easier, enhances adherence among patients, and ensures stable drug concentrations," he stated. "This once-a-week injection has the potential to make a significant impact."
Levodopa has traditionally been considered the primary treatment for Parkinson's disease. It passes through the blood-brain barrier and converts into dopamine a substance that decreases in supply with Parkinson's disease. However, its brief duration in the body requires individuals to consume it several times daily. This can result in variations in managing symptoms and raise the likelihood of not following the treatment plan properly.
Carbidopa is included to assist. It prevents levodopa from being metabolized prematurely within the body. As a result, a greater amount of the medication reaches the brain, which helps minimize adverse effects such as vomiting and enhances treatment success.
Related Stories
・ A new study reveals that smell and vision may detect Parkinson's disease several years before symptoms appear.
・ CRISPR enables researchers to discover important genes involved in the progression of Parkinson's disease.
・ Individuals suffering from sleep apnea have an increased likelihood of developing Parkinson's disease.
A detailed examination of how the system operates
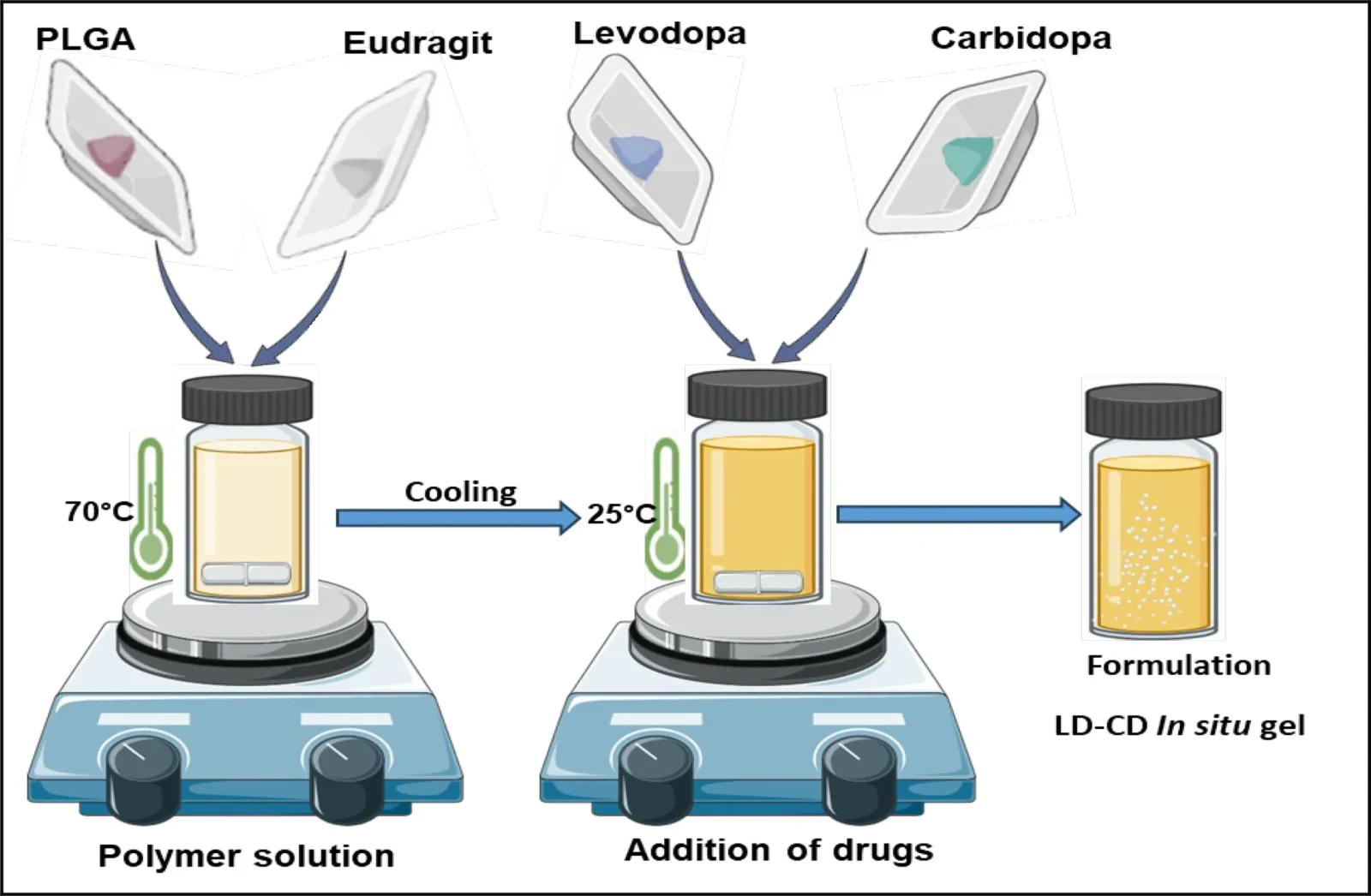
The injection-based system combines PLGA (poly-lactic-co-glycolic acid), an FDA-cleared biodegradable material widely recognized, with Eudragit L-100, a polymer responsive to changes in acidity. Upon administration, the mixture creates an implanted structure within the body, gradually delivering medication without requiring surgical intervention for placement or removal.
A UniSA doctoral candidate named Deepa Nakmode states that the design took many years to develop. "Following several years of dedicated research, it's very satisfying to witness our breakthrough in long-acting injections for" Parkinson’s disease arrive at this point. "Our innovation has now been submitted for an Australian patent," she stated.
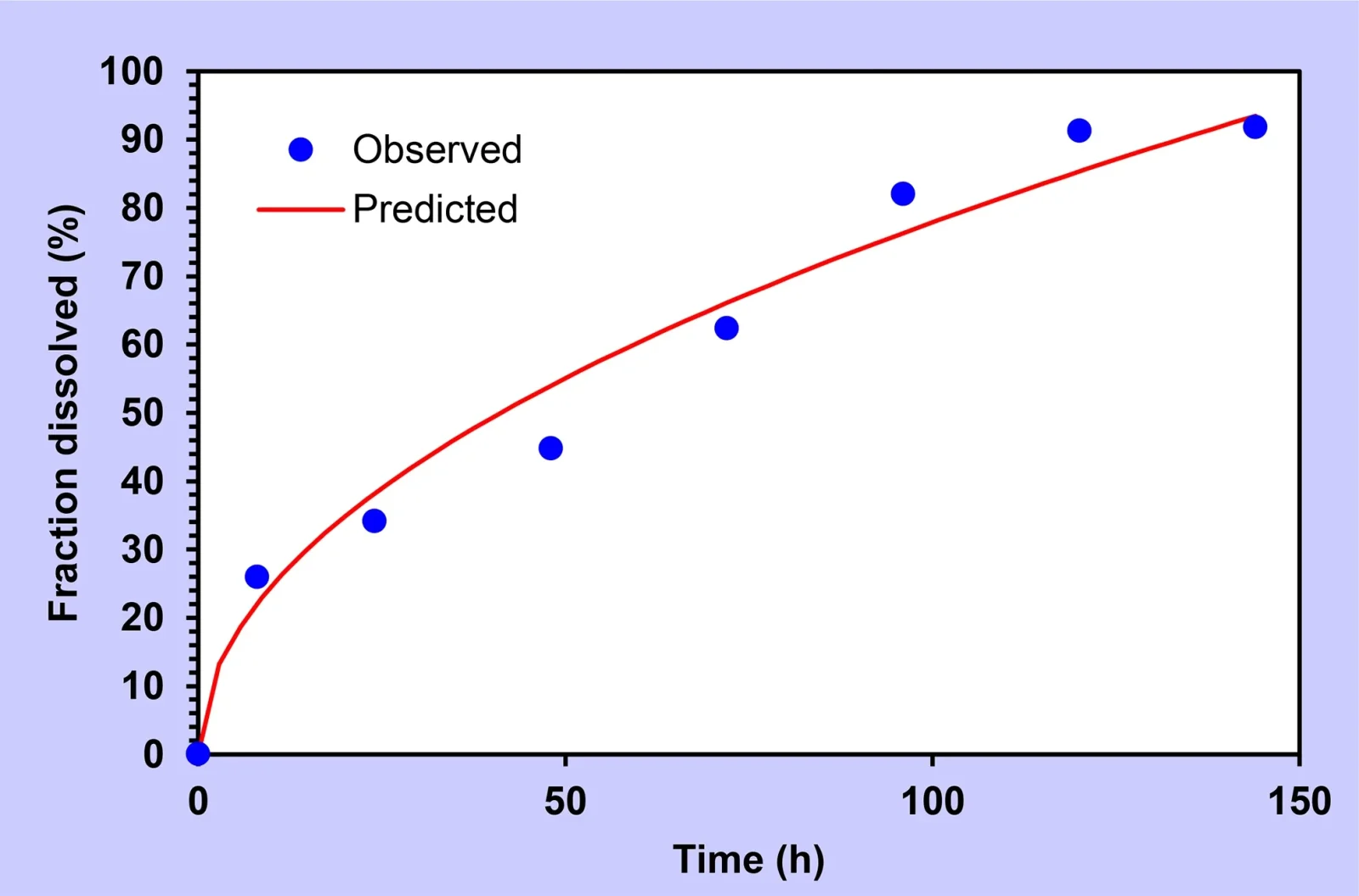
The enhanced formulation contains 26% PLGA and 6% Eudragit L-100. Experimental research demonstrated that within seven days, over 90% of the levodopa and 81% of the carbidopa were released. In the initial 24 hours, approximately one-third of the medication was delivered, aiding rapid alleviation of symptoms at an early stage.
Studies also revealed that the implant degraded by more than 80% within seven days. Notably, there were no indications of toxic effects in cellular testing, and the injection could be administered with a typical 22-gauge syringe, resulting in minimal discomfort and ease for most individuals.
Addressing challenges through present-day therapies
Parkinson’s is a progressive brain disorder resulting from the death of dopamine-producing cells. Although there is currently no cure, available therapies focus on alleviating symptoms. The standard approach involves taking pills orally. However, this method has drawbacks such as needing to take medicine often, inconsistent medication effects, and a significant risk of forgetting a dose.
Previous iterations of long-acting tablet formulations provided relief for certain individuals, yet they did not entirely address the issue. They were unable to fully prevent fluctuations in medication levels, and they still necessitated at least one dose per day.
This newly developed injection meets that requirement by ensuring steady medication delivery, increasing ease of use, and possibly boosting effectiveness. quality of life Reducing the dosage frequency from several times daily to once a week via an injection represents a significant advancement in treating Parkinson's," Professor Garg stated. "It's not only about enhancing drug delivery methods; it's about truly bettering patients' quality of life.

What makes in-situ implants distinctive?
Long-acting injections have become increasingly common in managing long-term illnesses due to their ability to enhance adherence to medication regimens and minimize adverse reactions. In-situ forming implants provide distinct advantages over alternatives such as solid implants or microparticles.
Not like solid implants, which might need surgery To add or remove, in-situ implants are introduced as a liquid that turns into a solid once inside the body. This approach eliminates the necessity for heat, additional ions, or surgical procedures. It functions through a process called solvent exchange: following insertion, the body's fluids come into contact with the implant, prompting the polymers to firm up and gradually deliver the medication.
PLGA was selected for this research as it is commonly employed in medication delivery because of its biocompatibility and adaptability. It degrades spontaneously within the body, enabling researchers to adjust its dissolution rate precisely. The group utilized a 50:50 combination of lactic and glycolic acids with a lower molecular weight, promoting quicker degradation—perfect for a once-weekly release.
Incorporating Eudragit L-100 enhanced the regulation of drug release. This pH-responsive polymer dissolves at a pH of 6 or above, making it appropriate for muscular or subcutaneous injection The group experimented with different proportions of PLGA and Eudragit to find an optimal combination of release speed and ease of injection. A particular formula, named P5, emerged as particularly promising.
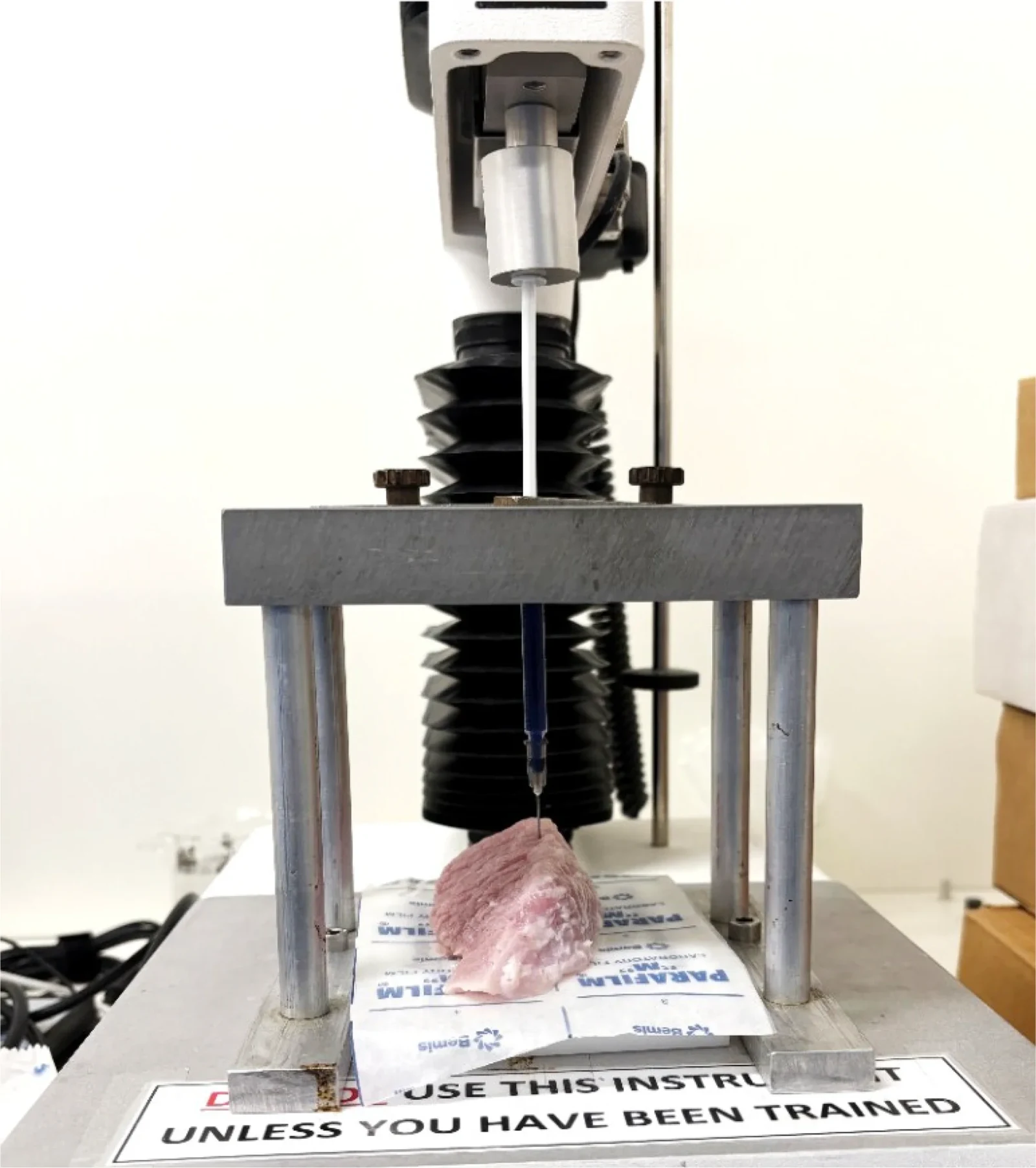
Testing revealed the finished product had a smooth consistency and acted like a Newtonian fluid (it moves smoothly), and needed approximately 33 newtons of force to administer. This is comfortably within the limits used in typical medical procedures.
Positive laboratory findings and subsequent actions Excellent test outcomes and following measures Favorable diagnostic results and future plans Successful lab evaluations and upcoming procedures Promising experimental data and next course of action Effective testing results and planned follow-up Solid analytical outcomes and continuing steps Good performance metrics and next phase of work Consistent with expectations, moving forward Validated results and proceeding actions
Scientists employed laboratory experiments along with computer simulations to examine how the medication functions. They estimated a maximum concentration (Cmax) of 399.3 nanograms per milliliter after 24 hours, and a cumulative drug exposure (AUC) of 26,505.5 ng/mL extending indefinitely. These findings indicate consistent delivery of the drug during the week, provided complete absorption occurs.
The gel system offers multiple benefits. It administers significant amounts of medicine without requiring surgical intervention, eliminates the need for big needles, and is simple to manufacture. These advantages make it appropriate not only for treating Parkinson’s disease but also for various chronic conditions that require ongoing treatment, like diabetes cancer, long-term discomfort, and illnesses.
Then, the UniSA group intends to initiate clinical testing and is seeking collaborations to introduce the product into the marketplace. Should it succeed, this innovation might substitute several daily medications with a single weekly injection, paving the way for novel methods of administering drugs within neuroscience and more.
A more expansive outlook for prolonged-release injections
The area of long-lasting injections has grown significantly in recent times. Pharmaceutical firms have explored different methods of administration, such as oil-based suspensions, liposomes, microneedles and implants. However, in-situ implants continue to be particularly promising as they enable higher drug capacities, adjustable release profiles, and easier delivery methods.
Earlier studies primarily concentrated on microspheres and solid implants, which faced challenges such as limited medication capacity, uneven distribution, and the necessity for surgical extraction. In-situ systems address many of these issues and offer a simpler, more convenient approach. Various commercially available products have already adopted comparable techniques, such as Atridox and Eligard.
The formula developed by UniSA could soon be added to this list, providing fresh optimism for individuals facing the challenges of everyday medicine.
Note: The article mentioned above was provided by The Positive Aspect of Current Events .
Enjoying stories like this? Get The Positive Aspect of News' mailing list .

Posting Komentar untuk "New Weekly Injection May Revolutionize Life for Parkinson's Patients"
Please Leave a wise comment, Thank you